Cell and gene therapies are innovative treatment methods that enable curative approaches for severe, previously incurable diseases. In addition to hematopoietic stem cell transplantation, therapies using genetically modified cells have also been approved as advanced therapy medicinal products (ATMPs) in the USA and Europe in recent years. These CAR-T cell therapies, in which the patient's own T cells are modified with chimeric antigen receptors (CAR), have so far been used almost exclusively in cancer therapy.
CAR T-cell therapy
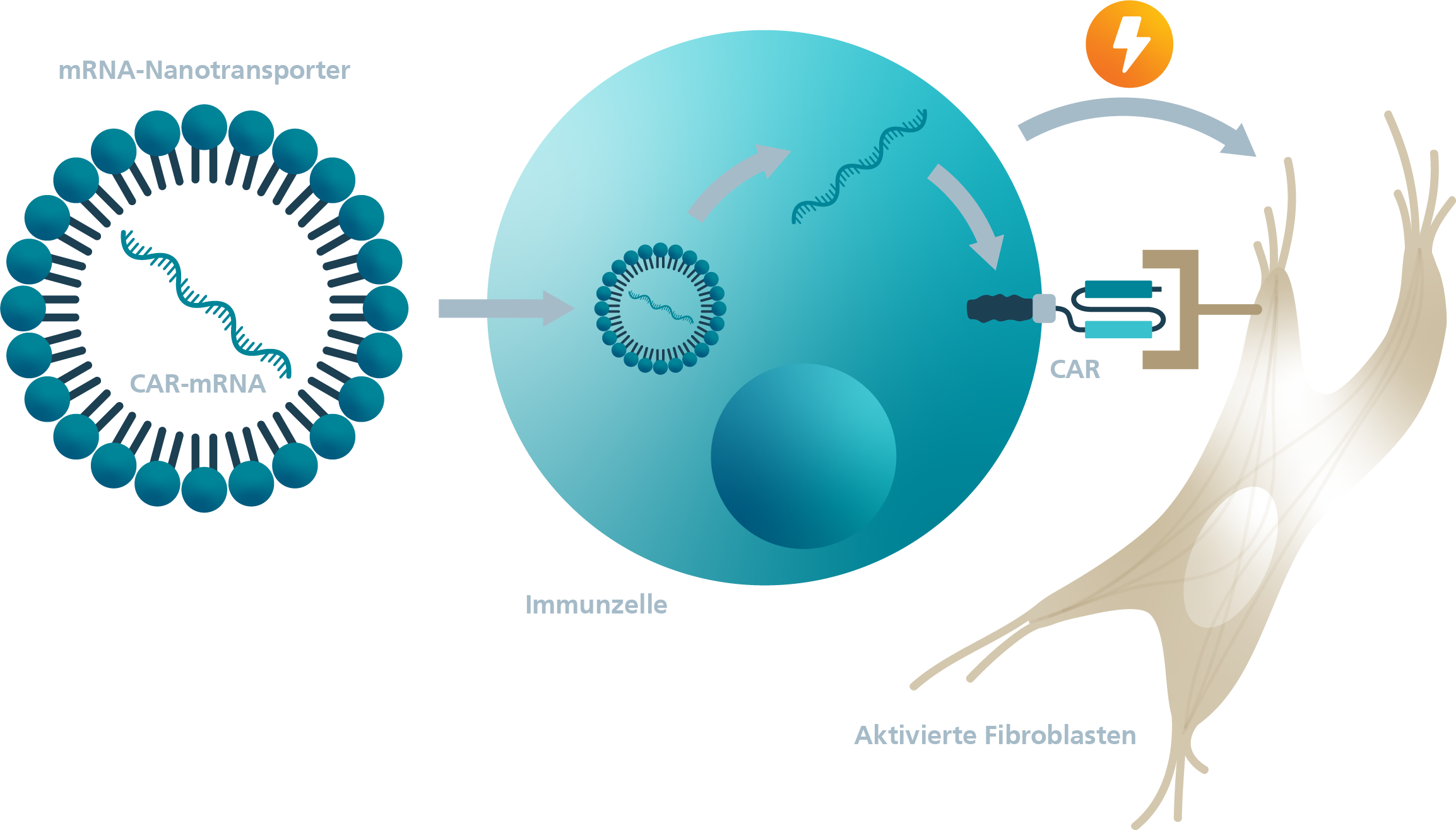
Approved CAR-T cell therapeutics, as well as the vast majority of new CAR-T cells in development, are based on the stable genetic modification of patients' own cells using viral vectors. Since CAR-T cell therapy is a very young method, the long-term consequences due to off-target effects, for example, have been scarcely studied. Furthermore, persistent CAR-T cells cause severe side effects. An alternative to stable modification is the temporary modification of cells by means of a messenger RNA (mRNA) coding for the CAR protein.
A transfer of the therapeutic approach of CAR-T cell therapy to infectious diseases, autoimmune diseases and fibrosis is state of the art in current research. In fibrotic diseases, the stromal-immune cell axis plays a crucial role. Therefore, activated misdirected fibroblasts represent an important target for therapies. Stably generated CAR-T cells against activated fibroblasts have been successfully used to reduce fibrosis in preclinical studies.
 Fraunhofer Cluster of Excellence Immune-Mediated Diseases
Fraunhofer Cluster of Excellence Immune-Mediated Diseases