“Toxicology in 4D” – 4D-Workshop IV of the Fraunhofer Cluster of Excellence for Immune-Mediated Diseases CIMD young scientists program
On November 23, 2020, the second virtual event of the young scientists program took place as part of the 4D workshop series of Fraunhofer CIMD.
The workshop was organized and conducted by the Fraunhofer Institute for Toxicology and Experimental Medicine ITEM. It focused on toxicology (in vitro and in vivo) within preclinical research and drug development. The participants consisted of employees of the core institutes of the research cluster (Fraunhofer IME, ITEM and IZI) as well as other Fraunhofer institutes (Fraunhofer IAIS, IAP, IGB, IGD, IKTS and IPA) and the MHH Hospital.
Prof. Dr. Norbert Krug, head of Fraunhofer ITEM and board member of Fraunhofer CIMD, opened the workshop with a welcoming speech for the workshop participants. Prof. Dr. Armin Braun, Head of Preclinical Pharmacology and Toxicology, gave an exciting presentation of the field of work of Fraunhofer ITEM.
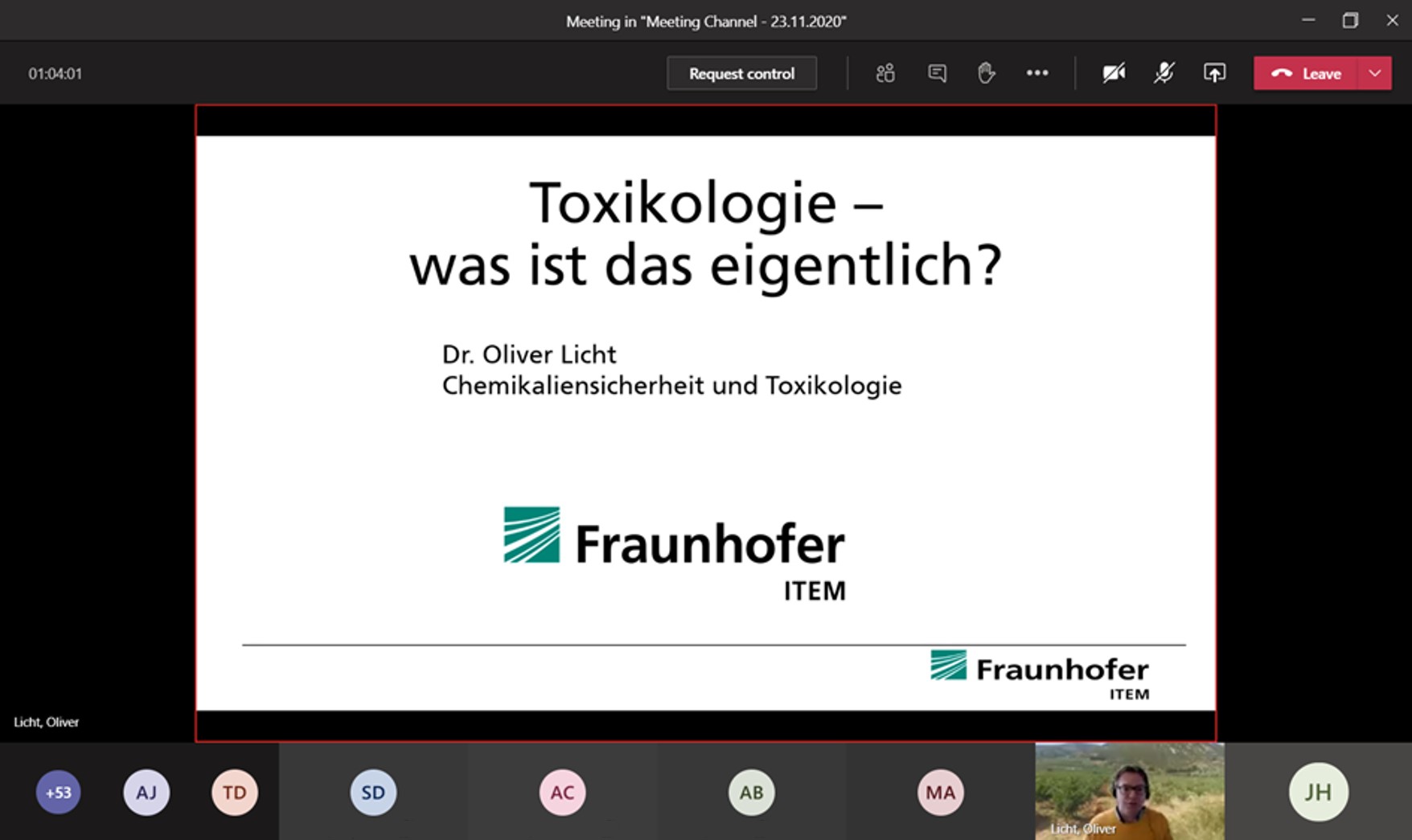
Afterwards, Dr. Oliver Licht, Department of Chemical Safety and Toxicology at Fraunhofer ITEM, explained in his presentation »Toxicology - what is it actually?«" the basic terms of toxicology, the use of data, the application of the 3r principle, and the basic principles of risk assessment.
After a short coffee break, Dr. Henning Weigt, Coordinator of the Business Unit Drug Development at Fraunhofer ITEM, gave an exciting insight into the preclinical phase, explaining in particular the non-clinical requirements in drug development. Prof. Dr. Theodor Doll, Coordinator of the Business Unit Translational Biomedical Engineering at Fraunhofer ITEM, then went into the basic safety requirements for medical devices, taking a closer look at the new EU Medical Device Regulation and the challenges it poses. This was followed by a presentation by Dr. Sylvia Escher, Head of Department of In-silico Toxicology at Fraunhofer ITEM, on the use of databases in risk assessment.
After the lunch break, the participants met in one of the four offered peer groups (Drugs, Data, Devices and Diagnostics), depending on their interests. Here, questions about the morning lectures or questions they had brought with them could be discussed with other participants and the respective moderator of the peer group. This was followed by two exciting case study presentations. The presentation »From the research laboratory into humans - Development of an RNA-based therapy« by Dr. Dorothee Winterberg, Head of Department of Preclinical Toxicology and Animal Laboratory at Fraunhofer ITEM and the lecture »Titanium dioxide as (nano)particle - from sun cream to food additive to the intense discussed inhalation toxicity« by Dr. Annette Bitsch, Division Director Chemical Safety and Toxicology at Fraunhofer ITEM.
The event was topped off with a keynote speech by Prof. Dr. Ulrich Kalinke, Managing Director of TWINCORE, Centre for Experimental and Clinical Infection Research. In his lecture "Initial testing of monoclonal antibodies in humans: Lessons from the TGN1412 accident" he explained very excitingly the challenges of preclinical investigations.
This interesting and informative event was a successful virtual continuation of Fraunhofer CIMD's young scientists program for networking and collaboration beyond the current projects.
 Fraunhofer Cluster of Excellence Immune-Mediated Diseases
Fraunhofer Cluster of Excellence Immune-Mediated Diseases