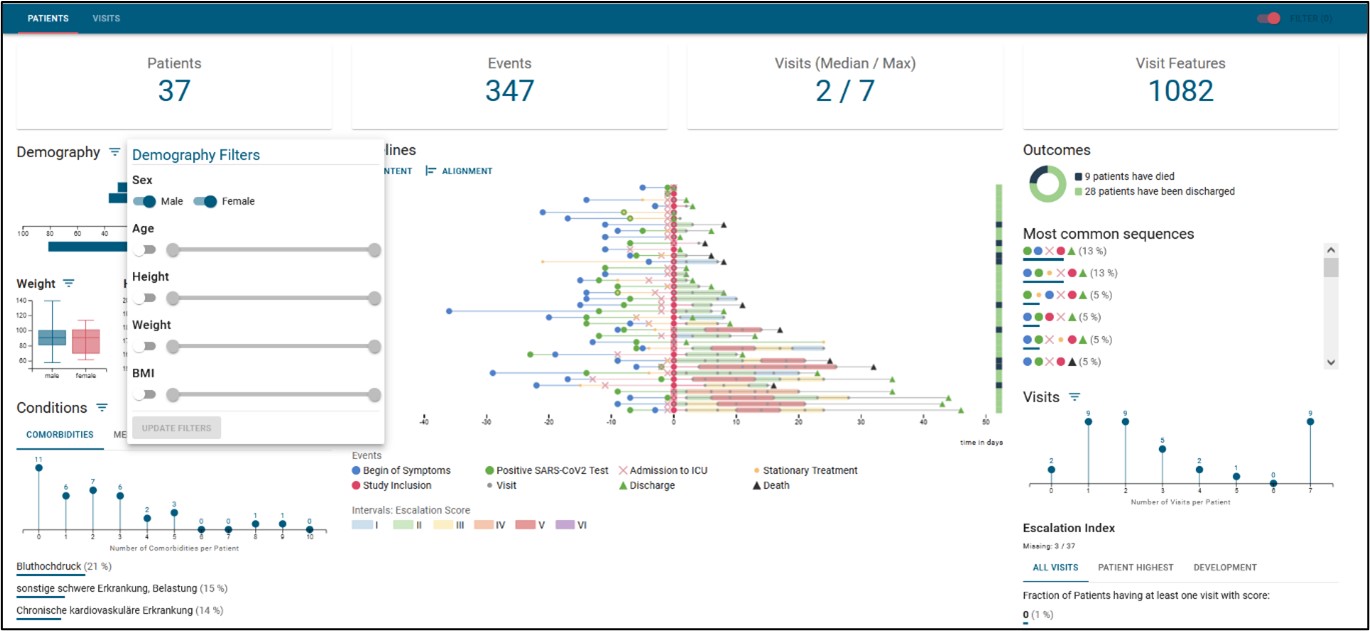
Potential pathway of an RNA-based drug

Innovative therapeutics based on ribonucleic acid (RNA) have become known to a broad public in the course of vaccine development. However, RNA-based vaccines are only one example of their application. In many organ diseases (for example, lung, heart, liver), altered gene expression signatures are known to influence disease progression. This is where RNA therapy comes in to provide leverage on pathophysiological processes.
Targeted RNA-based therapies
Understanding gene expression signatures altered in disease is key to developing a targeted RNA-based therapeutic strategy. In particular, the field of medical informatics and bioinformatics is an important component for the identification of key switch molecules and builds a bridge between patient data and application-oriented research.
The dovetailing of large clinical datasets, molecular biology data, and functional training of organ dysfunction has been demonstrated in many ways. Molecular RNA structures thus provide a prerequisite for the development of novel targeted RNA therapeutics.
Development of an RNA-based drug
The RNA Therapeutics platform will map a potential pathway that an RNA-based drug will follow from discovery to clinical use. Bioinformatic models will be used to select disease-associated RNAs that can be modulated via various RNA technologies. The RNA therapeutics will be transferred into protective encapsulation technologies (nanoparticles), qualitatively verified, and transferred into appropriate preclinical model systems to investigate therapeutic safety and efficacy.
Advanced RNA-based analytical methods to determine pharmacokinetic pathways complete the portfolio.
The processed proof-of-concept for RNA-based therapy development can be transferred to many other disease indications. In particular, the development of an appropriate encapsulation technology can be supportive here.
Synergistic effects will be achieved via the harmony of bioinformatics, in vitro/ex vivo models and the specific requirements of the encapsulation technologies. The platform therefore has a strong interdisciplinary character with dynamic control for future application models.
Another important development step will be the focus on RNA therapeutics production. This platform will act as a general realization platform for RNA-based therapies. Starting with small synthesis scales, RNA production will be available in larger quantities and incorporated into appropriate therapeutic strategies. Furthermore, the development of specific encapsulations/nanotechnology will be key for targeted therapy.
Outlook
Building on the results of the RNA therapeutics platform, further research activities in the field of RNA production and targeted nanoparticles will be necessary to optimize RNA-based drugs for therapeutic application. We are in intensive exchange with other Fraunhofer sites and external partners on this and see convincing potential for a new class of drugs in a broad clinical application.
 Fraunhofer Cluster of Excellence Immune-Mediated Diseases
Fraunhofer Cluster of Excellence Immune-Mediated Diseases